Abstract
Introduction: Recentreal-world data for multiple myeloma (MM) treatment patterns and outcomes are limited, particularly for patients with refractory or relapsed MM. As the MM treatment landscape evolves, it is important to understand how new treatments are integrated into patient treatment patterns. The aim of this study was to examine patient demographics, clinical characteristics, treatment patterns, and survival among Medicare patients with MM following exposure to daratumumab (DAR), an immunomodulatory agent, and a proteasome inhibitor (PI).
Methods: This was a retrospective database analysis utilizing the Centers for Medicare and Medicaid Services claims data during the study period of January 1, 2016, through December 31, 2018. The start of the study period was chosen to align with DAR market entry in the United States (FDA approval November 2015). Medicare patients, diagnosed with MM, and with existing claims for DAR, an immunomodulatory agent, and a PI (tri-exposure) were eligible for inclusion. Index tri-exposure was achieved once a patient had been exposed to all 3 MM treatments, regardless of their sequence. The index date was the first observed claim for any MM regimen (index line of therapy [LOT]) following tri-exposure. Patients were required to have ≥6 months of continuous enrollment prior to the index date (baseline period). Therapies given after the index LOT were defined as post-index therapy. Patient data were assessed until health plan disenrollment, death, or end of study period, whichever occurred first.
Results: There were 1336 Medicare patients with MM who met the inclusion criteria. Of these patients, the mean age (standard deviation [SD]) at the index date was 71 (8.5) years and 705 (52.8%) were male. The Southern United States showed the largest representation of patients in this population (n=471, 35.3%). The top baseline comorbidities included respiratory infections (98.3%), osteoarthritis (86.8%), anemia (86.9%), hypertension (78.1%), dyslipidemia (66.5%), chronic pain/fibromyalgia (51.9%), acute or chronic kidney disease (44.5%), cardiac arrhythmia (45.81%), and neutropenia (47.98%).
The mean (SD) number of days from the index date until the end of index LOT was 48.7 days (12.0). Among 949 patients (71.0%) who had post-index therapy, the mean (SD) time from the last observed claim for index LOT to the beginning of the post-index therapy and duration of post-index therapy was 51.8 (44.2) days and 57.8 (16.0) days, respectively. The median (range) duration of follow-up time from the index date was 223 (3─886) days. During the study follow-up period, 571 patients (42.7%) died, and the median (range) number of days from the index date until death was 173 (3─873) days.
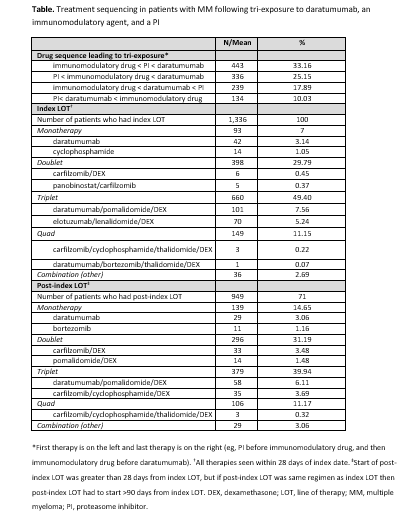
While there was variation in treatment sequencing leading to tri-exposure, the most frequent tri-exposure sequence was an immunomodulatory agent < PI < DAR (33.2% [See Table for overview of treatment sequencing]). Among patients who received index LOT following tri-exposure, 49.4% had triplet therapy and 29.8% had doublet therapy. The most common index LOT regimens were triplet: DAR/pomalidomide/dexamethasone (DEX; 7.6%), elotuzumab/lenalidomide/DEX (5.2%), and DAR/bortezomib/DEX (4.9%). The most common post-index LOT regimens were triplet (39.9%): DAR/pomalidomide (6.1%), carfilzomib/cyclophosphamide/DEX (3.7%), DAR/bortezomib/DEX (3.5%), and DAR/lenalidomide/DEX (3.5%). During follow-up, 52.8% of patients were retreated with DAR, 79% with PI, and 77.3% with immunomodulatory drugs.
Conclusions: This study suggests wide variation in treatment sequencing and regimens after tri-exposure to DAR, an immunomodulatory agent, and a PI. Triplet regimens were predominant treatment after the tri-exposure and following the index LOT. Retreatment with the same agents was common. Among those who died, survival was often less than 1 year following tri-exposure. These results highlight the need for new treatment options in triple-class refractory MM settings.
Funding: GSK (Study 213462)
Boytsov: GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company. Cyhaniuk: STATinMED Research: Current Employment. Leung: STATinMED Research: Current Employment. Wang: GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company. Hogea: GlaxoSmithKline, paid employee: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Mudumby: STATinMED Research: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal